My mind has finally settled on doing contemplations like these instead, so that I can effectively synthesize instead of letting all my knowledge (or the lack thereof) remain stagnant in my head.
Check below the break for this contemplation!
Ah, life. What is it anyway? We keep hearing that term, yet only a few of us have dared to let their minds roam and seek for answers. For some, they could care any less. But for the sake of this post, what is life? Think about this question, get off, then come back here when you have the answer. Or ignore this altogether. Besides, this question is not the focus of this post.
Anyway, to define it using gobbledygook, life is basically a summation of all biochemical reactions that work together to produce a self-producing output without external intervention, as long as the necessary reagents are present. (Now this is a reductional definition, meaning that we are just a bunch of molecules, so this is not an appropriate definition.) In high school biology, it involves organization, metabolism, regulation, response, movement, growth and development, reproduction, and evolution. But of course, the definition goes beyond the realm of biology. In fact, life can be defined based on emotion, work output, success, activities, etc. After all, life is not all spent in studying the study of life itself.
But for plants like these, life is just about growing towards maturity and reproducing.
But first, you might think, "Why did I click this?" It may be due to curiosity, or it may be attributable to opinion or thought searching in the deep fabric of web. It may actually because of some trivial quest that involves nitpicking of articles, as to look for errors or disagreements or deviations from your opinion. Whatever the reason might be, it does not matter. You all came here to witness how an amateur college student explains how this mystical force came into being.
There are two ideas on this: abiogenesis and special creation. To be honest, I see the latter as a product of human imagination and curiosity involving the figure of perfection known as The Creator or God. It is the "explanation" first thought by people from the ancient times who just got accustomed to their surroundings. It can also be a poetic or a figurative interpretation of the beginning.
The product of human imagination, rather than something that will contradict empirical data.
I do not see anything wrong with this, nor see anything that makes this true while falsifying scientifically-found methods. After all, beliefs are distinct from knowledge; therefore, these are not meant to clash against each other, nor are atheists and devouts encouraged to fight with each other. If you think otherwise, then review the difference between beliefs and knowledge.
But for the sake of this contemplation, let us just focus on empirical evidence discovered by different scientists: abiogenesis. Whether you believe it or not, I do not have a problem with your opinion. But this is the inference of scientists based on observed evidences, so there is truth in this theory, unless an empirical evidence disproves it, which may be unlikely.
Disclaimer: Some parts may involve hardcore science (terms or vocabulary). Please bear with me, and do not worry, pastel-colored equines might be found along this post to entertain or to annoy you. Or, just click away. It's up to you.
Wait, you are still here? Okay then, time to put you to sleep. Hush now quiet now...
What was the Earth when it was created 4.6 billion years ago? A hot ball of molten rock.
Meteorites are bombarding it as if it's an obstacle that must be eliminated by flying rocks. Gases like ammonia, hydrogen, methane, carbon monoxide, carbon dioxide, nitrogen, and hydrogen sulfide are present. A red moon, along with shooting meteors that tend to crash directly on Earth's surface, can be witnessed. No, silly, you should not be imagining yourself in this environment, because the temperature is just too high for you to survive there. You're gonna die there in seconds.
This isn't home...
Before we proceed, did you think of oxygen as a gas in the primeval atmosphere? If so, then sorry, you are wrong. Oxygen was not present. In fact, it only appeared when cyanobacteria emerged, and that is when photosynthesis became a process. But before photosynthesis, there was no oxygen at all.
Okay, let's continue. So what if we have these gases in the atmosphere? What now? Let us apply the principles of chemical kinetics. High temperatures, due to lightning and the earth not yet cooling down, result to higher kinetic energy, which causes reaction rates to be higher, thereby making synthesis reactions possible. Another causative agent is the ultraviolet radiation from the Sun, which induce homolytic cleavage of molecules, causing them to be more reactive in the form of radicals that can react with other gases. As a result, reactions like nucleophilic substitution (and whatever crap) occur. Methane may react with molecules like ammonia or hydrogen, and if you are looking for the exact mechanism of the reaction, then sorry, but there are chemical equations of this:
- 1) CO2 → CO + O (decomposition of carbon monoxide)
- 2) CH4 + 2[O] → CH2O + H2O (radical attack of elemental oxygen on methane)
- 3) CO + NH3 → HCN + H2O (reaction of ammonia with carbon dioxide)
- 4) CH4 + NH3 → HCN + 3H2 (reaction of ammonia with methane; BMA process)
The molecule CH2O is a formaldehyde, which is the simplest carbohydrate. HCN, which is hydrogen cyanide, is a precursor of amino acids, which are made through the Strecker synthesis:
- CH2O + HCN + NH3 → NH2-CH2-CN + H2O
- NH2-CH2-CN + 2H2O → NH3 + NH2-CH2-COOH (glycine, the simplest amino acid)
Overall, simple organic molecules like amino acids are made. Regarding synthesis of nucleotides, sugars, and fatty acids, we will not delve into that, but these molecules are presumed to be made as well. Anyway, how did we get to know that monomers got formed? Here:
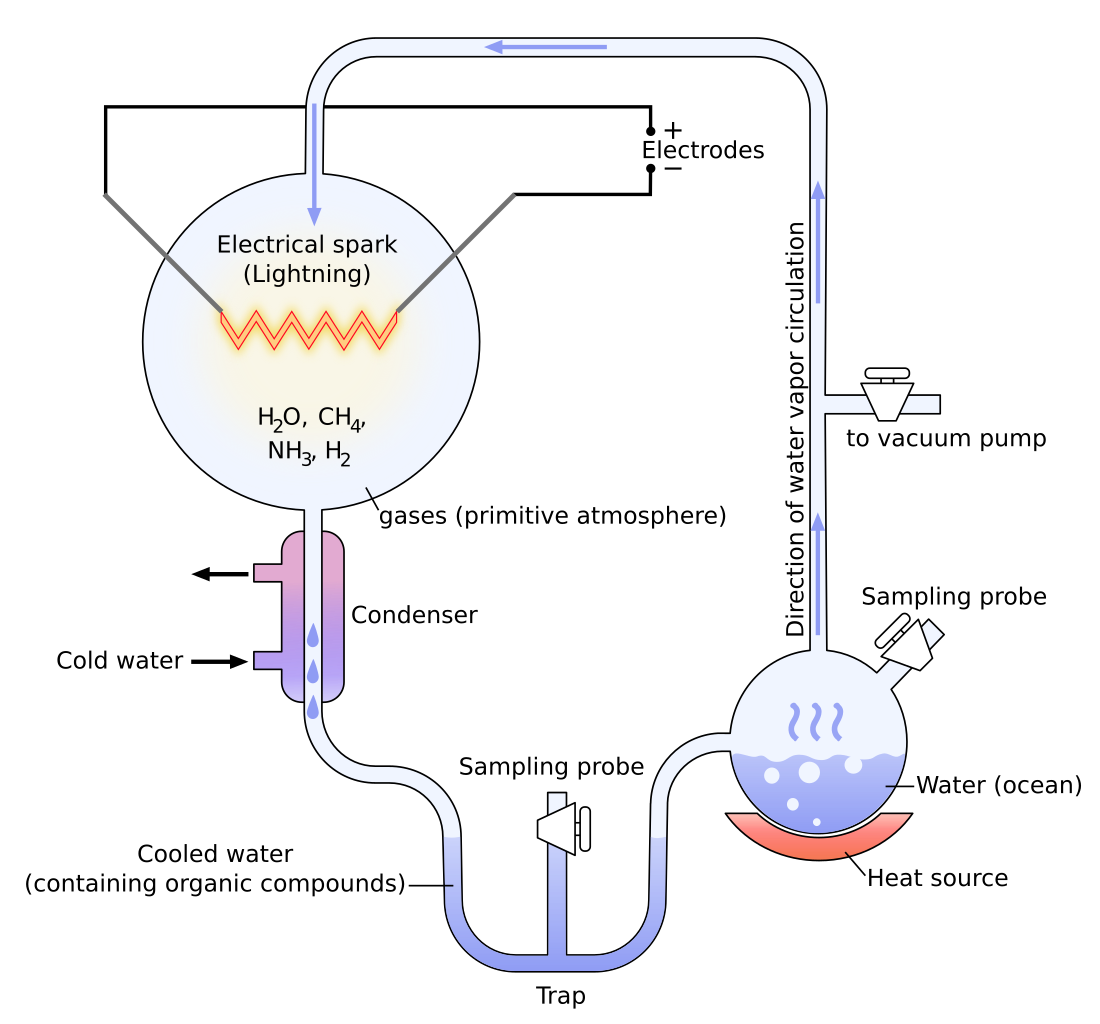
Behold, the experiment that confirmed it all!
What is this, you might ask. It's the Miller-Urey experiment, designed to test Alexander Oparin's synthesis by simulating conditions that mimic the conditions of early Earth. As you can see here, there are connected flasks. The water on one flask represents the "ocean". Due to high temperatures, water tends to evaporate. In another flask, there are gases that represent the early atmosphere, along with the electric spark that represents lightning. Because of the high temperature of lightning, it makes reactions of gases possible, leading to small organic molecules. As time passes, water condenses and accumulates in "primordial soups" that contain organic molecules. Evidence like this does not lie!
What happens next? Reactions involving inorganic molecules continue to occur, resulting to more biochemical monomers getting produced, along with other organic molecules. These monomers accumulate as time goes by, eventually forming "organic soups" all throughout the face of our planet.
As they accumulate, some of them react with each other in an unlimited number of ways. As long as they collide and they have the proper orientation, the product can form. In many cases, their collision is ineffective, which means that wrong portions collide with each other, resulting to no combination, or, if there's any, then the product would be unstable. But in some cases, collisions lead to a bigger product, or a polymer. But if only two monomers collide, then it is just a dimer, but of course, the chain can grow, resulting to a chain of monomers or a polymer. This process is called polymerization.
This isn't exactly what happened, as the molecules aren't the amino acids that we know, but this diagram just illustrates that monomers combine to form polymers. Also, don't mind the "Stereospecific Living Anionic Polymerization."
Because polymerization is endothermic and results to decreased entropy (due to the decrease in the number of molecules), one might think that it is impossible, according to the Gibb's Free Energy law. But take note of the conditions of primordial Earth. High temperatures. Therefore, these reactions can occur, albeit in a slow manner. In fact, it might take thousands, or millions of years! Case in point: Miller-Urey experiment. Time has passed, and no proteins. It can be attributable to the lack of catalysts, which would have sped up reactions to just seconds or minutes, but there were no proteins yet to catalyze polymerization.
But somehow, polymerization still occurred. Thanks to high temperature. Through a variety of organic reactions, a mixture containing more complex organic molecules was formed. But because molecules can impact each other at varying angles, an unlimited collection of organic molecules can result. Some molecules might have formed, but they might have decomposed due of their instabilities. Others remained stable, due to their low potential energies and their relative stabilities. Some of these stable molecules are the biomolecules that we all know. Oligopeptide, nucleotide strands, polysaccharides, fatty acid chains, and more organic molecules.
Feeling tired? Here, have an adorable mare snuggling something. D'aww.
"I've never seen anything so, adorable!"
Let's get back to work. Why these oligomers? (Gr. oligos, few + Gr. meros, part) Why not fully-functional proteins or DNA? Remember, this is just a soup of organic molecules. No functions yet; they are just lying around doing practically nothing but reacting with other molecules. Yes, they react with molecules, but not similar to how fully-functional proteins interact with each other or how DNA commands protein synthesis. It's far from that. For now, this is just polymerization that leads to only short fragments, and not long functional ones. It would take millions of years before long polymers are made, unless catalysts are present that can make polymerization reactions possible.
Awhile ago, I mentioned that there is no oxygen yet. It is an advantage. An advantage indeed. Because if oxygen were there, combustion reactions would occur. Take note that these reactions degrade molecules into simpler ones. Not to mention that these are exothermic and result to high entropy, so they easily occur. Therefore, the natural tendency of molecules in the presence of oxygen and high temperatures is to "burn" and decompose. But the apparent goal is to make complex molecules, not break them, so the presence of oxygen disrupts this goal. Good thing there was no intervening force that would have prevented this molecule-building.
Steps so far:
1. Gas reaction to form simple monomers
2. Accumulation of monomers
3. Polymerization of monomers to form oligomers, then polymers
4. ??? (on the next post)
Thank you for trying to read this or sleeping over this! Don't worry, there will be more torture coming up, detailing about how could polymer organic molecules actually give rise to life. I apologize if I did not answer the question of how life actually arose. Yet. But just in case you are so excited (like Pinkie) that you want to know now, here it is:
Gas --> Monomer --> Polymer --> Fully-functional biomolecules (and catalysts) --> Unicellular organisms
This is all that I will discuss for this contemplation series; I may not delve into multicellular life. Also, if you have any questions, just ask in the comment section.
References:
I am too lazy to put my sources here, but I acknowledge the fact that almost all the things presented here are just borrowed from scientific writings, so they are not mine.
References:
I am too lazy to put my sources here, but I acknowledge the fact that almost all the things presented here are just borrowed from scientific writings, so they are not mine.
Disclaimer: Some of these info might not right at all, or just conjured up in my mind. Remember, this is what an amateur college student knows (and pretends to know). Please bear with me, and I apologize if many assumptions are made. Besides, I do not need to pass a paper detailing that, so I injected equines and other stuff. Don't worry, if I have time in the future (after I've read a biochemistry book), I might make a revision to make it more accurate. Also, this post might be edited.